Photon correlator for the study of fluorescent dye molecules
The goal of this project is to develop an instrument to study the spatial correlations amongs fluorescent dye molecules as they diffuse around in solution. It turns out that the spatial correlations can be reconstructed if the arrival times of the fluorescent photons can be recorded. This technique goes by the name of fluorescence correlation spectroscopy and we are hoping to design and build our own instrument to be able to do this. Much of the preliminary work has been done thanks to the extraordinary efforts of two very talented students Chloë Gulbronson ‘23 and Paige Bulgrin ‘22. Their outstanding work not only resulted in a grant being awarded to our lab to carry this work forward, but was also presented at the UWSP Research Symposium in May 2022.
Brief description and layout
The idea behind fluorescence correlation spectroscopy is to illuminate a very dilute solution of molecules with a tightly focused beam of one wavelength and capture the emitted fluorescent photons of a slightly longer wavelength. Our implementation of this idea is based on a classic paper ``Fluctuation correlation spectroscopy for the advanced physics laboratory" by Rieger, Rocker, and Nienhaus, Am. J. Phys., 73, 1129 (2005) with some slight modifications in how the photon correlator is calculated within the software. The figure below shows the layout of our experiment. The beam from a green 532 nm diode pumped solid state laser passes through a spatial filter and beam steering mirrors before being incident on a dichroic beam splitter. This beam splitter guides the beam through a high NA objective onto a sample cell filled with a dilute solution of molecules. The fluorescent photons emitted are captured by the same objective and are guided onto a single photon sensor using the appropriate beam optics.
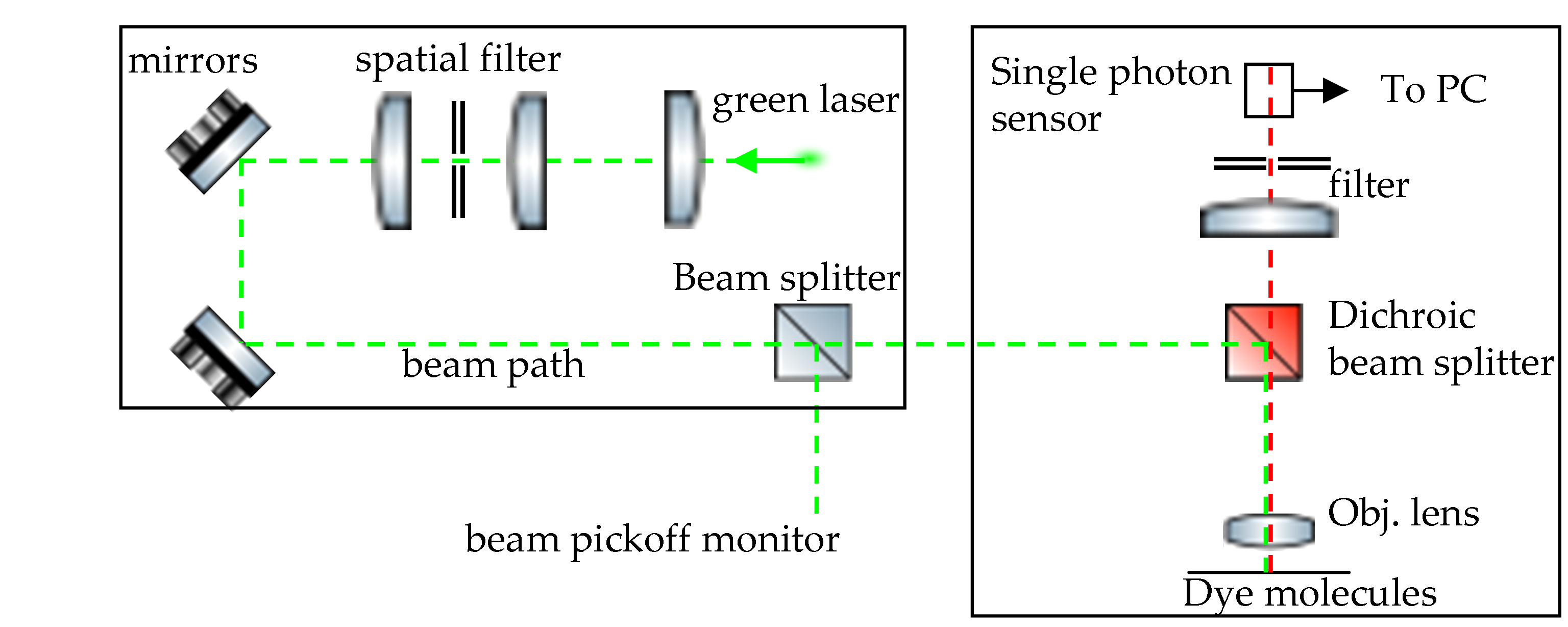
Our single photon sensor is a 6 mm square silicon photomultiplier (SiPM), chosen mainly for its ease of use and the use of low voltages required for its operation. The output of the SiPM is sent to a timing single channel analyzer (SCA) NIM module. The timing SCA produces a 500 ns wide 5 V pulse if the lower level discriminator threshold is satisfied and these clean digital pulses are sent to a NI 6612 PCIe timer counter card that records the arrival time of each photon event. The arrival times are then converted into a correlation spectrum using our own custom software. A
poster describing these aspects of our initial work is available.
Current work
We are using this instrument to address two questions.
- What is the correlation time and the diffusion constant of rhodamine molecules in solution? This project is led by Chloe Gulbronson ('23) and Cody Korth ('23). The challenge here is to get the measurement volume small enough that the physics is dominated by the fluctuations. This usually means a measurement volume of about a few fL and extremely dilute solutions. The students have just finished the construction of new sample cells which will be compatible with our 100x oil immersion objectives. The cells can easily be loaded with about 12 uL of rhodamine solution and we can prepare solutions ranging from a concentration of 1 mM all the way down to 1 nM. We believe the 100x objective will help focus the laser spot down to a diffraction limited spot size of roughly 1 um.
- To measure the fluorescence lifetime of rhodamine molecules using the method of time correlated single photon counting (TCSPC)? The challenge here is that these lifetimes are at best a few ns long. This project is led by Mitchell Imlah ('23). A new redesign of the emission beam optics path has just been finished and new components have been ordered. This redesign will allow us to align a confocal aperture with the single photon sensor much more easily. A circuit to generate ns pulses is also being constructed and a refurbished time-to-amplitude converter NIM module necessary for implementing the TCSPC technique is about to be tested.
